Kamil Firudinovich Abdullaev a,*, Ekaterina Valerievna Orlova a, Manish Kumar Yadav a, Evgeny Alexandrovich Vasilyev b, Mikhail Arkad’evich Mokhirev a, Kseniya Sergeevna Gileva a
a “Central Research Institute of Dentistry and Maxillofacial Surgery” (CRID), Timura Frunze street, building 16, Moscow, Russian Federation
b “The Maxillofacial Hospital for Veterans of Wars”, Lesteva street, building 9, Moscow, Russian Federation
* Corresponding author. CRID, Timura Frunze street, building 16, Moscow, 119021, Russian Federation.
https://doi.org/10.1016/j.jpra.2018.02.001 2352-5878/© 2018 The Author(s). Published by Elsevier Ltd on behalf of British Association of Plastic, Reconstructive and Aesthetic Surgeons. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
|
A R T I C L E I N F O |
A B S T R A C T |
|
Article history: Received 9 June 2017 Accepted 5 February 2018 Available online 27 February 2018 |
Background: Technological advancement in medical science is constantly innovating solutions to the varied and complex challenges of surgery. Digital diagnostics and prospective microsurgery are rapidly evolving. Three-dimensional (3-D) imagery and computed tomography (CT) scanning can determine accurate dimensions of many defects. Subsequently, a thorough understanding of microvasculature and application of microsurgical techniques allows modelling of flaps to obtain an accurate transplant resulting in an aesthetic outcome following the very first operation. Methods: Two patients with Parry-Romberg syndrome and one patient with haemifacial microsomia (Goldenhar syndrome) were treated with anterolateral thigh (ALT) flaps to restore facial volume, contour, and symmetry. In each case, a different approach in planning and performing the intervention was applied: The patient in the first case had a full-thickness ALT flap transplant with significant overcorrection. The patient in the second case had reconstruction with a partially thinned ALT flap guided by a clinically formed template made per manual measurements. The patient in the third case had reconstruction with a precise primary thinned ALT flap with a template made according to data obtained from superimposed 3-D photographs and CT scans. Results: All flaps survived. In cases 1 and 2, a corrective intervention was required to achieve acceptable facial symmetry. In case 3, a very good aesthetic result was achieved immediately after the first operation. Conclusions: Digital methods of 3-D analysis offer great opportunities in creating a precise operative plan, and modern surgical techniques make it feasible to implement it intra-operatively. Overall, these methods shortened the rehabilitation time by avoiding further revision surgeries. © 2018 The Author(s). Published by Elsevier Ltd on behalf of British Association of Plastic, Reconstructive and Aesthetic Surgeons. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). |
|
Keywords: ALT Perforator flap Facial haemiatrophy Parry–Romberg syndrome Goldenhar syndrome 3-D planning |
Introduction
The pioneer microsurgeons3,5,11 who made eminent contributions to plastic surgery would admire contemporary plastic surgeons,1,2,4 who not only use perforator flaps in the free style form from any area of the body,6,7 but also perform thinning of flaps,8–10 to acquire the required thickness and thereby improve results of reconstruction at the very first surgery. Since 1984, when Song first demonstrated the use of the anterolateral thigh (ALT) flap,11 the pos- sibility of microsurgical auto-transplantation has increased. Currently, microsurgical auto-transplantation is a standard approach in the treatment of patients with severe facial asymmetry, such as those affected by Parry–Romberg syndrome.12–15 The treatment of patients with Parry–Romberg syndrome types 3 and 4 (Guerrerosantos et al.)16 and severe cases of Goldenhar syndrome with haemifacial mi- crosomia, involves a comprehensive approach including correction of bony deformities and volume- contouring of tissue defects. This paper illustrates the authors’ experience in treating 3 patients with severe haemifacial microsomia and haemiatrophy, with emphasis on the features of soft tissue flap transplantation. However, scrutiny of the adjuvant stages such as: orthognathic surgery and silicone implantation were not done.
Materials and methods
Patients
From 2015 to 2017 at the Central Research Institute of Dentistry and Maxillofacial Surgery Moscow, three patients aged between 25 and 32 years were treated. Two patients were diagnosed with Parry– Romberg syndrome (type 4), and one patient was diagnosed with Goldenhar syndrome (haemifacial microsomia). All patients had presented with a history of unsuccessful surgical corrections (e.g., lipofilling, silicone implants, and polyacrylamide gel injections) at several other institutions.
Preoperative assessment
According to the methods of Zhou et al., Kimata et al., and Xu et al.,3–5 the perforator vessels on the thigh were identified using a hand-held audio Doppler scanner. In addition, colour duplex Doppler scanning of the thigh and neck was performed.
The methods of planning and intraoperative techniques evolved in technical precision from the first case to the third.
Surgical technique
An incision was made anterior to the tragus in the parotid-masseteric region in the subcutaneous layer above the superficial muscular aponeurotic system (SMAS) to avoid damage to the facial nerve branches. The skin within the atrophied zone was mobilised to form a pocket for flap placement. The recipient vessels were identified using the submandibular approach.
In all cases, the ALT flap was raised from the distal to the proximal end. During flap mobilisation, the perforators were identified as they exit through the fascia lata. When a thinned flap was required it was raised within the superficial fascial layer; and, the easiest way to identify perforators was in between the smaller superficial fat lobules and large deep fat lobules.10
After the flap was isolated on the identified perforating vessels, the fascia lata was incised, and further dissection continued in the subfascial space. The perforators led proximally to a common source, and dissection of the vascular pedicle continued until the required length was achieved.
The flap was then transferred to the face, and stabilised with holding sutures until the microsurgical anastomosis was completed.
The precision of the flap sculpting gradually improved from the first to the third case. Individual differences are described for each patient.
Observations
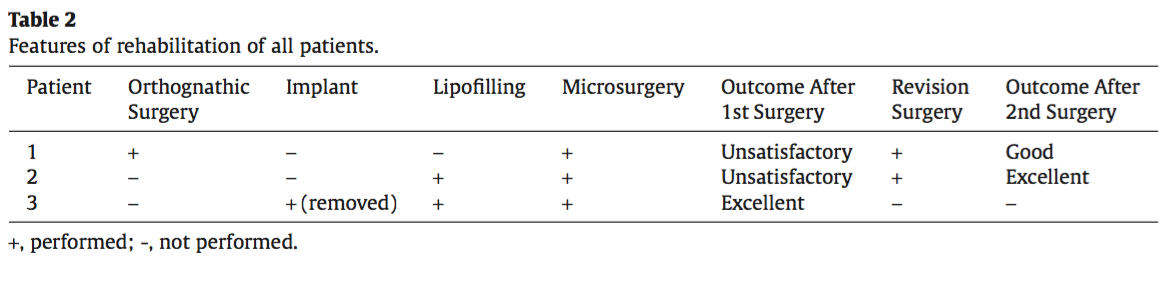
The atrophied area ranged from a minimum of 15.0 × 6.0 cm to a maximum of 21.5 × 11.0 cm. In the first and third case, the flap was raised on musculocutaneous perforators, whereas in the second, it was on the septocutaneous perforator. The vascular pedicle was between 5 and 8 cm in length. The operating time varied according to the complexity of flap modelling (ranging from 6 h in the first case, up to 11 h in the third). Features of the surgeries are provided in Table 1.
Results
Cases
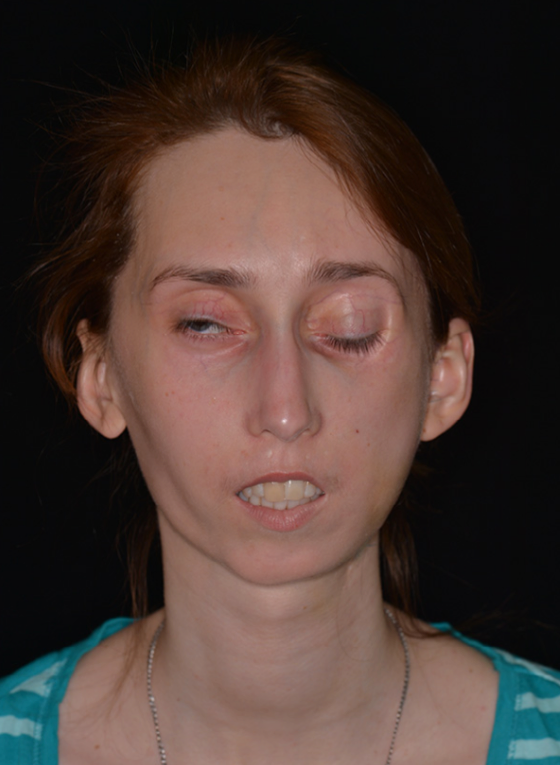
Patient oneIn 2015, Ms. S, a 25-year-old woman, presented with right-sided haemifacial microsomia mani- fested as: anotia, malocclusion class 2 (Angle’s classification), asymmetric mandibular deformation, zygomatic bone hypoplasia, and haemifacial atrophy. She was diagnosed with Goldenhar syndrome. The treatment was performed in two phases. During the first phase, in March 2015, a series of op- erations were performed to correct the malocclusion (distraction osteogenesis in the right mandibular ramus, median palatal expansion, and mandibular bilateral split sagittal osteotomy and maxillary Le Fort 1 osteotomy) (Figure 1).
Figure 1. First patient, preoperative frontal view.
Figure 2. First patient, postoperative frontal view.
Figure 3. First patient, post revision surgery at 8-month follow-up, frontal view.
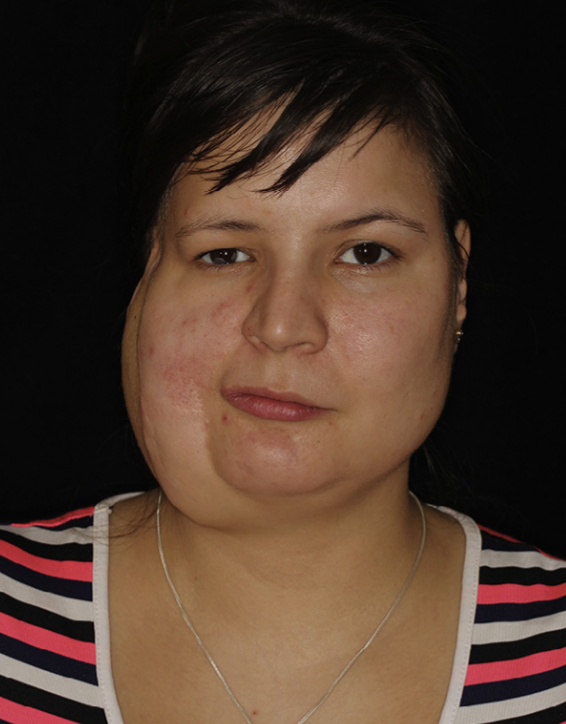
In 2016, Ms. L, a 28-year-old woman, presented with progressive left haemifacial atrophy. The patient had disease onset at 4 years, and the atrophy progressed until the age of 15, when she was diagnosed with Parry–Romberg syndrome. In 2007, on stabilisation of the syndrome, lipofilling was done to restore the facial symmetry; however, fat resorption occurred (Figure 4).
Figure 4. Second patient, preoperative frontal view.
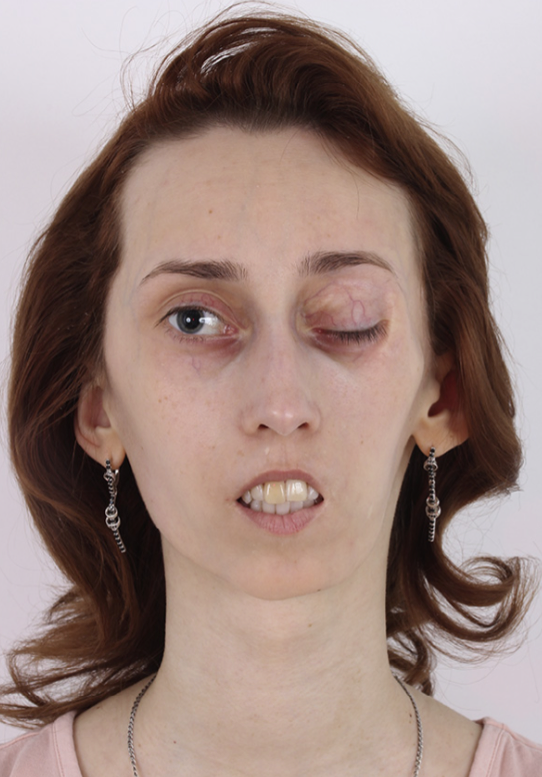
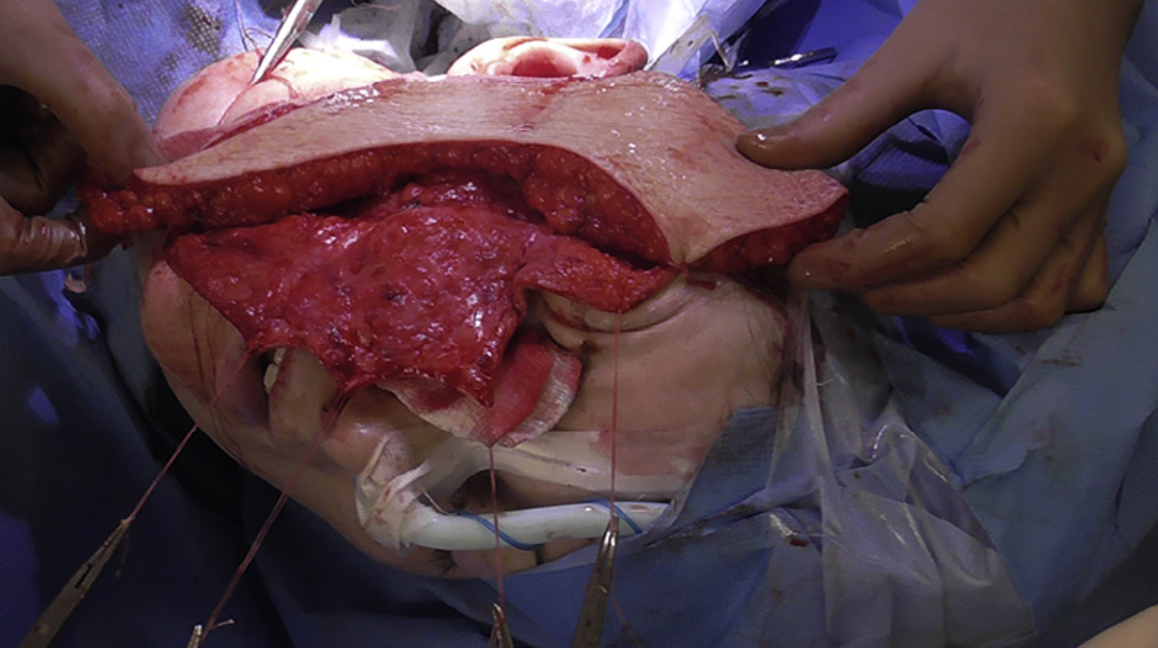
Figure5. Secondpatient, intraoperative left lateral view of the face. Extended preauricular incision with an individually modelled de-epithelised adipocutaneous ALT flap with skin paddle placed over the affected region.
Figure 6. Second patient, postoperative frontal view.
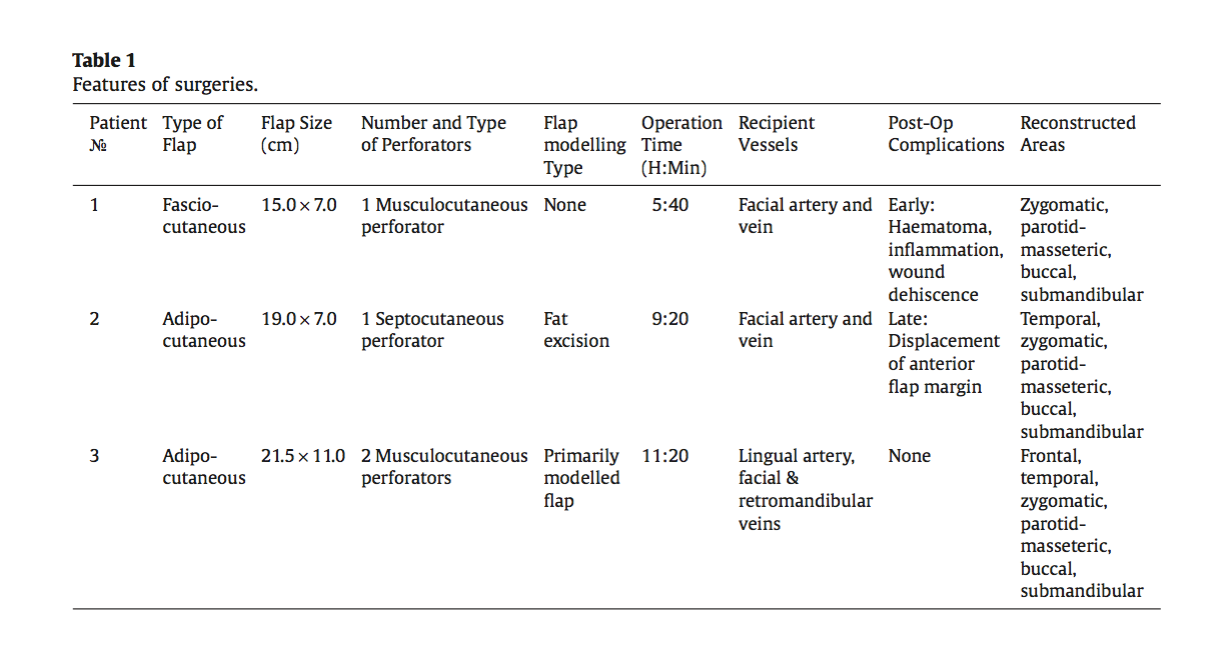
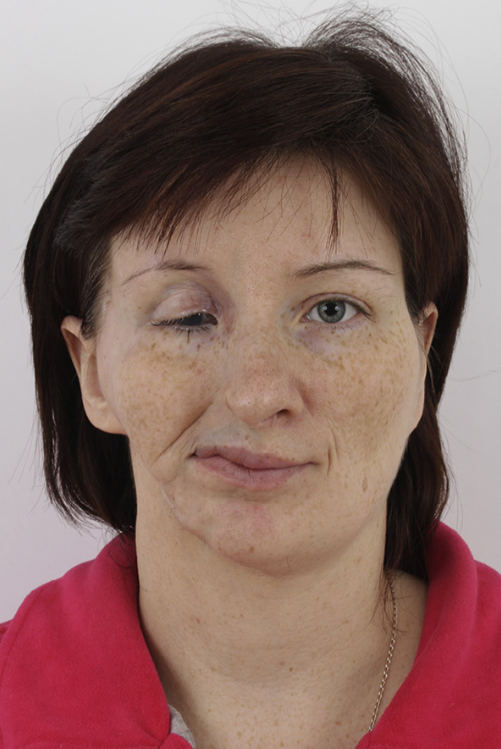
In 2017, Ms. L, 32-year-old woman, presented with right haemiatrophy, hypoplasia of the right zygomatic bone, asymmetric deformation of the jaws, and malocclusion class 2 (Angle’s classification). She was diagnosed with Parry–Romberg syndrome (type 4). Atrophy began when the patient was 6 years of age, and reached peak manifestation at the age of 13 years, stabilising at the age of 21 years. At the age of 20, in another hospital, she underwent silicone implants in the right zygomatic and temporal areas. Following an inflammatory response, the implants were removed and, subsequently, multiple corrective interventions were performed at other institutions, but they were ineffective. In 2017, at the age of 32, she came to our hospital, the Central Research Institute of Dentistry and Maxillofacial Surgery (CRID), Moscow (Figure 7).
Figure 7. Third patient, preoperative frontal view.
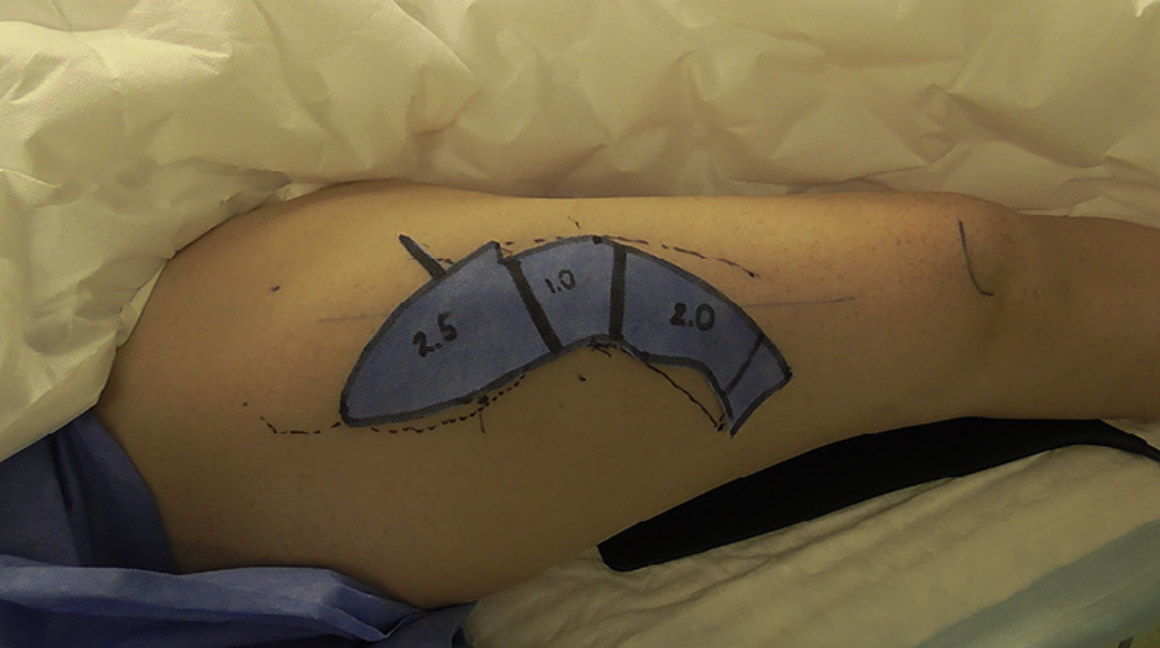
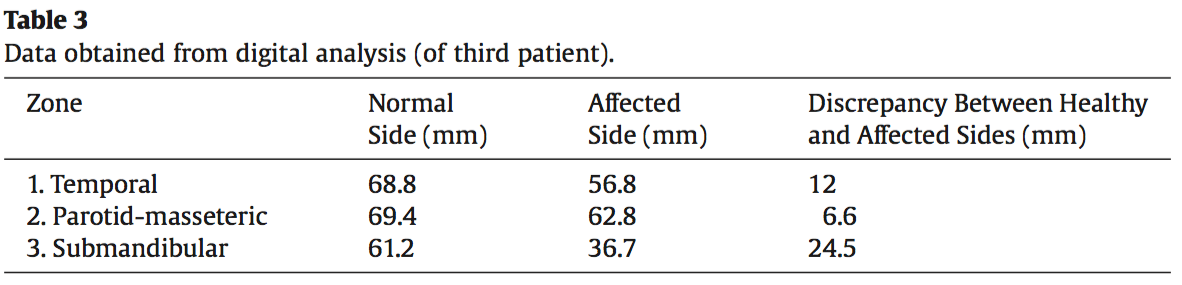
Figure 8. Third patient, intraoperative template in situ on right thigh. Data noted on the template to guide the appropriate thickness of the ALT flap in different areas, as obtained from digital analysis (see Table 3).
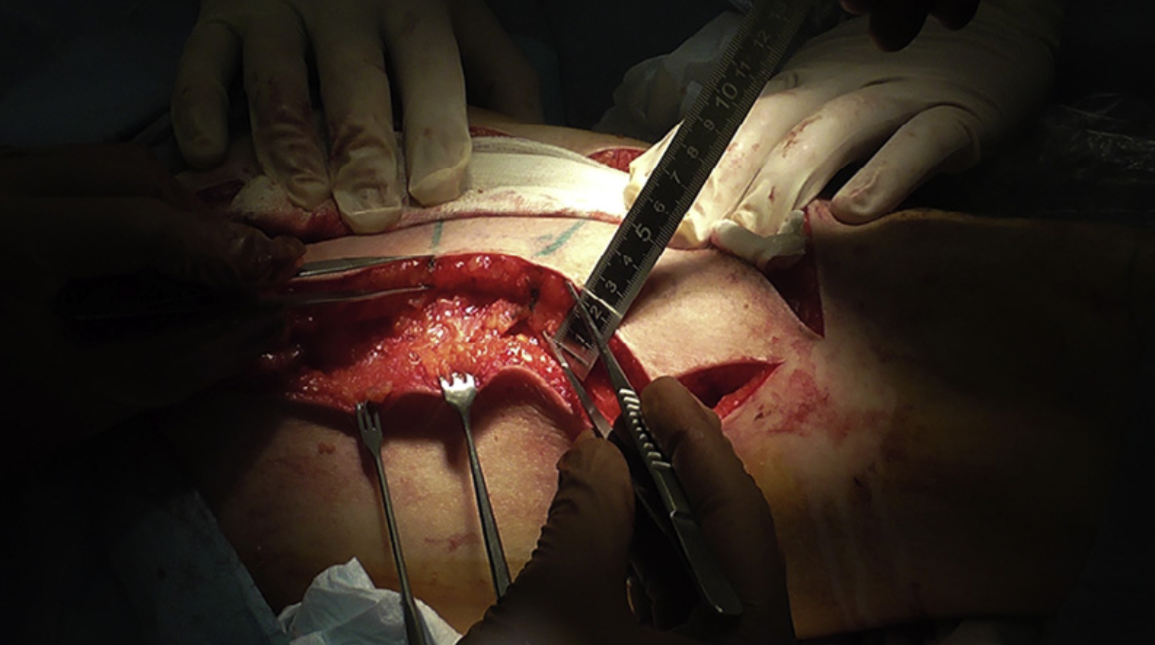
Figure9. Thirdpatient,intraoperativevaryingthicknessofALTflapharvest.Harvestofaprimary-thinnedadipocutaneousALT flap in progress. Ruler and forceps demonstrating the varying thickness in different areas of the ALT flap as per the template (see Figure 8).
Figure10. Thirdpatient,intraoperativerightlateralviewofthefacewithflapinsitu.Theprimary-thinnedandmodelledALT flap placed over the recipient site after revascularisation (prior to de-epithelialisation). The varying thickness of the ALT flap is evident, as it matches the natural contour of the affected region.
Figure 11. Third patient, postoperative frontal view.
Discussion
ALT flaps have a high level of variability in the source and type of perforating vessels.6 However, the anatomical variations did not prevent the ALT flap from gaining popularity3,4,17 for soft-tissue defect reconstruction. The blood supply to the skin of the lateral thigh is provided by perforators that typically arise from the descending branch of the lateral circumflex femoral artery (LCFA). Depending on their relation- ship with the vastus lateralis and rectus femoris muscles, these perforators may be either septo- or musculocutaneous.
The principal role of the vascular plexuses in the blood supply of skin flaps has been recognised.18,19 Alkureishi L.W. et al.20 reported that the communicating vessels between the plexuses do play a sig- nificant role in the blood supply of the flap. Further refinement was demonstrated by Saint-Cyr M. et al.,21,22 proving that the viability of the flap remained high even after radical thinning. There are two methods of obtaining a thin flap: one is the thinning of the flap after harvest by excision of fat, and the other is harvest of a primary-thinned flap.12 Each method has its own advantages and limitations. After harvest, thinning of the flap is technically easier because, while raising the flap over its fascia lata, the perforating vessels become more apparent. However, profi- ciency in the harvest of a primary-thinned flap with desired thickness at the shortest operating time is technically challenging.
The latest techniques on “hot and cold spots” of “perforator distribution”10,23 are designed to sim- plify and accelerate the method of obtaining a primary-thinned flap. With some experience, this method allows the surgeon to raise the flap faster and minimise damage to the donor site by preserving the lateral cutaneous nerve of the thigh and its branches.
Adipofascial and adipocutaneous ALT flaps
Gang Chai et al., Loubin Si et al., and Li Teng et al.13–15 mentioned the advantages of an adipofascial flap in eliminating asymmetry of facial contours, leaving an acceptable scar at the donor site with the possibility of complete skin preservation. Also, a harvest of de-epithelised flap preserves the fascia lata and lateral femoral cutaneous nerve. Likewise, Ji, Y. et al.24 also mentioned the feasibility of trans- fixing the fascia lata to the underlying immobile structures at the recipient site. However, because of the inherent mobility of the deep subcutaneous fat over the fascia, the adipofascial flap is not adher- ent to the pocket created at the recipient site and, thus, results in flap instability and sagging. Also, in adipofascial flaps, postoperative shrinkage may reach up to 25%.14 With such a high degree of vari- ability, the feasibility of an accurate simulation in the third case would not be possible.
Adipocutaneous flaps consist of a superficial fatty layer with small fat lobules, which has a strong affinity to the dermis. Because of this strong bonding, fixation of the dermis to bony structures and temporal fascia appears more stable for flap fixation. Also, these fat lobules are more compact and dense, resulting in less postoperative shrinkage. This was one of the reasons why we chose an adipocutaneous flap for reconstruction. As the skin overlying the atrophy zone is of poor quality, it should be excised and, thus, the skin component of the adipocutaneous flap is necessary to replace the formed defect. However, the best aesthetic result was achieved with full de-epithelisation of the adipocutaneous flap. A major drawback of an adipocutaneous flap is a larger defect at the donor site.
Flap planning
Using modern methods of 3-D visualisation, detailed analysis of defects can be planned for a better restorative outcome. Gang Chai et al.14 demonstrated the advantages of laser scanning in comparison to 3-D photographs.
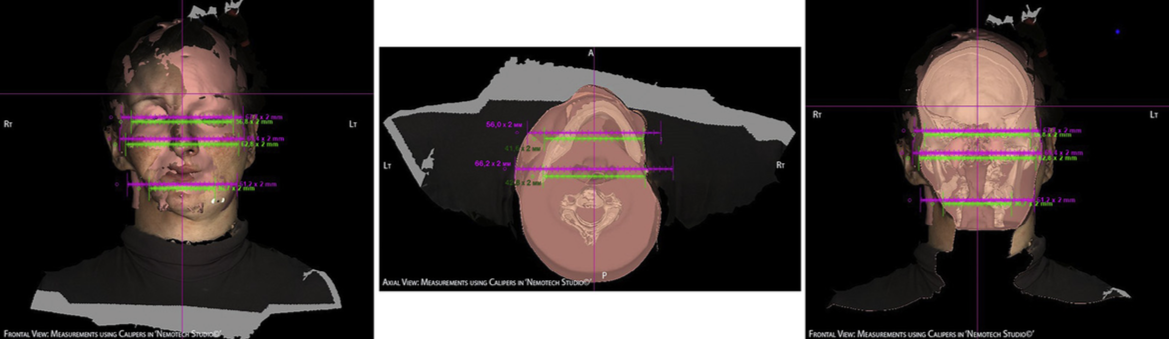
Clinically it is important to maintain the head in a natural head position (NHP)25 to have an accu- rate assessment of the soft-tissue volume in the submandibular and submental regions. For a precise measurement, CT scans and 3-D photographs should also be performed in NHP. We performed a thor- ough marking of the atrophied facial zone and pasted radiographic contrast markers (Gutta-Percha and Radiological Markers, IZI Medical Product) onto the patient’s face. Then, 3-D photographs and CT scans of the patient’s head were taken, and the acquired images were superimposed using the Nemotech Studio© software (Screen dump 1).
Screen dump 1. NHP Bone and Skin images.
Screen dump 2. Superimposed 3-D Photos and CAT Images in Nemotech Studio (green – affected side measurements; pink – healthy side measurements).
Flap fixation and drainage at the recipient site
In the second patient, a complication of flap displacement on removal of the transcutaneous sutures during the postoperative period led to improvisation of the technique. Accordingly, in the subse- quent case, the flap was fixed at five points: 1. the cartilage of the tragus, 2. temporal fascia, 3. periosteum of the zygomatic bone, 4. periosteum of the mandible, and 5. subcutaneous tissue of the subman- dibular region.
Given the large area of mobilisation and detachment, the placement of an active drainage below the flap gives the best results by preventing haematoma formation.26 Nonetheless, an additional drain- age above the flap was established to reduce the mobility of the overlying skin, thus avoiding the wobbling effect.
Lipofilling and silicone implants
In cases with minor soft-tissue asymmetry and deformities, lipofilling is considered the treat- ment of choice due to its relative simplicity, low rate of donor site morbidity, and minimal complications. However, in cases of severe haemifacial atrophy and microsomia, the poor vascularity and microcir- culation of superficial tissues is a major hindrance for lipofilling, as it often results in resorption of a significant amount of injected fat. In the second patient, two lipofilling procedures prior to flap trans- plantation resulted in an almost 100% resorption. However, lipofilling procedures performed post flap transplantation in the second and third patients showed retention of a major portion of the injected fat, as observed at 1-year post procedure. The reason for this phenomenon was the improved blood supply to the recipient area due to the transplanted vascularised tissue.13
Likewise, the advantages of silicone implants are the feasibility of precise sculpted implants and absence of donor site morbidity. However, the implants do not match the softness and texture of the subcutaneous tissues. Because it is securely fixed to the bone, the rationale would be to use it in re- storing symmetry of bony deformities, as it often provides better results than osteotomy and tissue transplantation do. When placing implants in the area of atrophy, especially in Parry–Romberg syn- drome (type 4), one of the major problems observed was the atrophied skin and subcutaneous structures with poor elasticity, thus making it quite challenging to create an adequate pocket for the implant with its probable consequence of inflammation and extrusion.
Conclusions
Modern 3-D visualisation techniques, together with the feasibility of selective flap thinning, can create favourable conditions for an ideal outcome in soft-tissue atrophy and haemifacial microso- mia. It was found that the conjunction of CT scan with 3-D photographs remained the most informative tool in analysing the differences in volume between the affected and healthy sides. Also, an in-depth analysis of the advantages and disadvantages of adipofascial and adipocutaneous flaps in choosing the preferred method of reconstruction was deemed necessary.
A positive aesthetic result of soft-tissue flap transplantation in volumetric contouring was found mostly dependent on the variables such as: determination of deficit in the affected zones, the use of flap modelling techniques, preplanning of fixation points to the underlying fixed structures at the re- cipient site, and possibility of maximum de-epidermisation of the applied adipocutaneous flap.
Successful treatment of patients affected with severe forms of haemifacial microsomia and pro- gressive haemiatrophy (types 3 and 4) was feasible with a planned comprehensive approach. It was also ascertained that lipofilling and silicone implants were most efficiently applied post microsurgi- cal transplantation, and not prior to the transplantation.
Conflict of interest
None.
Funding
None.
References
- Wei FC, Jain V, Celik N, Chen HC, Chuang DC, Lin CH. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109:2219–2226, discussion 2227-30.
- ChenH,TangY.Anterolateralthighflap:anidealsofttissueflap.ClinPlastSurg.2003;30:383–401.
- Zhou G1, Qiao Q, Chen GY, Ling YC, Swift R. Clinical experience and surgical anatomy of 32 free anterolateral thigh flap transplantations. Br J Plast Surg. 1991;44:91–96.
- KimataY,UchiyamaK,EbiharaS,etal.Anatomicvariationsandtechnicalproblemsoftheanterolateralthighflap:areport of 74 cases. Plast Reconstr Surg. 1998;102:1517–1523.
- XuDC,ZhongSZ,KongJM,etal.Appliedanatomyoftheanterolateralfemoralflap.PlastReconstrSurg.1988;82:305–310.
- ChangC-C,WongC-H,WeiF-C.Free-stylefreeflap.InjIntJCareInjured.2008;39:57–61.
- MardiniS,TsaiF-C,WeiF-C.Thethighasamodelforfreestylefreeflaps.ClinPlastSurg.2003;30:473–480.
- Kimura N, Satoh K. Consideration of a thin flap as an entity and clinical applications of the thin anterolateral thigh flap. Plast Reconstr Surg. 1996;97:985.
- KimuraN,SatohK,HasumiT,OtsukaT.Clinicalapplicationofthefreethinanterolateralthighflapin31consecutivepatients. Plast Reconstr Surg. 2001;108:1197.
- HongJP,ChoiDH,SuhH,etal.Anewplaneofelevation:thesuperficialfascialplaneforperforatorflapelevation.JReconstr Microsurg. 2014;30:491–496.
- SongYG,ChenGZ,SongYL.Thefreethighflap:anewfreeflapconceptbasedontheseptocutaneousartery.BrJPlastSurg. 1984;37:149–159.
- JinX,TengL,XuJ,etal.Anterolateralthighadipofascialflapfortherestorationoffacialcontourdeformities.Microsurgery. 2010;30:368–375.
- SiL,ZengA,QiaoQ,etal.Microsurgicalcorrectionofprogressivefacialhemiatrophyusingfreeanterolateralthighadipofascial flap. J Craniofac Surg. 2012;23(7 suppl 1):2051–2056.
- Chai G, Tan A, Yao CA, et al. Treating Parry–Romberg syndrome using three-dimensional scanning and printing and the anterolateral thigh dermal adipofascial flap. J Craniofac Surg. 2015;26:1826–1829.
- Teng L, Jin X, Wu G, et al. Correction of hemifacial atrophy using free anterolateral thigh adipofascial flap. J Plast Reconstr Aesthet Surg. 2010;63:1110–1116.
- GuerrerosantosJ,GuerrerosantosF,OrozcoJ.ClassificationandtreatmentoffacialtissueatrophyinParry–Rombergdisease. Aesthet Plast Surg. 2007;31:424–434a.
- Di Candia M, Lie K, Kumiponjera D, et al. Versatility of the anterolateral thigh free flap: the four seasons flap. Eplasty. 2012;12:21.
- Pearl RM, Johnson D. The vascular supply to the skin: an anatomical and physiological reappraisal—Part II. Ann Plast Surg. 1983;11:196–205.
- DanielRK,KerriganCL.Principlesandphysiologyofskinflapsurgery.In:McCarthyJG,ed.Plasticsurgery,Vol.1.Philadelphia: WB Saunders; 1990:275–328.
- Alkureishi LW, Shaw-Dunn J, Ross GL. Effects of thinning the anterolateral thigh flap on the blood supply to the skin. Br J Plast Surg. 2003;56:401–408.
- Saint-Cyr M, Schaverien M, Wong C, et al. The extended anterolateral thigh flap: anatomical basis and clinical experience. Plast Reconstr Surg. 2009;123:1245–1255.
- Saint-CyrM,WongC,SchaverienM,etal.Theperforasometheory:vascularanatomyandclinicalimplications.PlastReconstr Surg. 2009;124:1529–1544.
- AbrahamJT,Saint-CyrM.Keystoneandpedicleperforatorflapsinreconstructivesurgery:newmodificationsandapplications. Clin Plast Surg. 2017;44:385–402.
- JiY,LiT,ShamburgerS,etal.Microsurgicalanterolateralthighfasciocutaneousflapforfacialcontourcorrectioninpatients with hemifacial macrosomia. Microsurgery. 2002;22:34–38.
- SolowB,TallgrenA.Headpostureandcraniofacialmorphology.AmJPhysiolAnthropol.1976;44:417–435.
- Badyul PA, Sliesarenko SV, Sliesarenko KS. The efficacy of postoperative drainage of space under flap while reconstruction plastic surgery. Пластична реконструктивна і естетична хірургія. 2015;3:32–39.